Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Test videoLorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
HomeLorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
HomeLorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Home
CytoCell® FISH probes provide high intensity signals and minimal background to enhance detection and scoring accuracy. Pre-mixed liquid probes minimise the possibility of mixing errors.
CytoSure® is a leading provider of array and NGS products for research into cytogenetics, rare disease and cancer. Our NGS solutions benefit from having superior CNV calling with excellent concordance with the ‘gold standard’ microarray.
Discover more
The SureSeq™ haematology and solid tumour ranges encompass NGS panels allowing accurate detection of a wide range of aberrations implicated in various cancers. Next to our catalogue panels, you can choose from our regularly updated, expert-curated library of pre-optimised cancer content to create your ideal custom SureSeq myPanel™.

Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
HomeImproving upon existing SureSeq™ technology, this latest SureSeq NGS panel unlocks more efficient and expansive genetic profiling for CLL samples. The figure (above) illustrates the panel's uniformity and high depth of coverage, allowing the confident detection of [A] a SF3B1 exon 15 hotspot variant Lys700Glu with 4.8% allele frequency and [B] a TP53 exon 4 frameshift deletion (TP53 c.124del) with frequency 38.9%.
View ProductDeveloped in partnership with myeloid cancer experts to the latest WHO guidance, the panel can identify novel fusion partners — providing more comprehensive and informative analyses than possible using PCR-driven methods. The figure (above) shows the consistent and confident detection of MECOM overexpression in [A] serial dilutions of HNT-34 cell line as well as [B] research and commercial samples, including positive and negative controls.
View ProductOur latest and most advanced system for capture of targeted genomic regions and generation of NGS libraries. Delivering a 40% reduction in hands-on time compared to the previous workflow, increased accuracy and error correction with the inclusion of UDIs and UMIs and reduced workflow complexity for minimal risk of human error. The figure (above) shows the workflow.
View ProductThe kit generates NGS libraries suitable for the capture of targeted genomic regions using hybridisation. Delivering high performance with low duplication rates, high sequence quality and high percentage of on-target bases. The figure (above) shows how the kit delivers exceptional sequence quality scores even 150 bases into the read.
View ProductWorking in the R&D group at OGT has provided me with the opportunities to work within a great team whilst also working closely with colleagues across the commercial and computational areas. It is great to be a part of a team that develops exciting products for use by researchers all around the world.

Sandra Kachhia
Senior Scientist, OGT
To be working in R&D at CytoCell, is to be working in a truly unique, collaborative environment. Being a part of this team has been both exciting and rewarding; together we innovate new products whilst also investigating and improving existing products, ultimately to ensure only the best is going out to the world.
Fabiha Alam
Research Assistant, OGT
I am proud to be a member of Operations. As a team we are equally committed to helping and supporting each other in an encouraging environment, each of us strive to achieve our best potential, ensuring we provide high quality products. It feels incredibly rewarding to know the products we make are helping people and health facilities around the world.

Emma Stimson
Senior Product Scientist, OGT
I enjoy working within the Operations department because it is fast paced and we work in a close team who pull together to achieve a common goal.

Rebecca Richer
Production Supervisor, OGT
My placement with OGT has given me a unique and valuable insight into the NGS industry, all while learning so much from my wonderful colleagues and working on products that bring real benefits to researchers. There really is no substitute for a placement of this quality as an undergrad.

Tiffany Damon
R&D Placement Student, OGT
Becoming part of the Computational Biology team has meant I have experienced part of the essential role bioinformatics has within the healthcare industry. It’s been fascinating but challenging to collaborate with other members of R&D to work towards the delivery of new products with exciting potential.

Zachary Scurlock
Computational Biology Placement Student, OGT
I’m incredibly lucky to work in such a supportive environment with an amazing and talented team, where I feel valued and my opinions are always taken into account. Proud of a small team developing huge innovations in Cytogenetics.
Elena Orell
R&D Research Assistant, OGT
Working as a research scientist at CytoCell is a very rewarding opportunity. As part of a team, you advance a project that will have an immediate clinical applicability by developing a new technology or improving an existing product, that will be used to diagnose diseases in patients.
Alejandra Collazos
R&D Research Scientist, OGT
As a product manager at OGT I work on the crossroad of our products and customers. It is incredibly rewarding to work with my colleagues and our customers to accelerate cancer research, enabling smarter decision making in clinical care and improving human healthcare every day.

Kay Wiebrands
Strategic Marketing Manager SureSeq
Having spent the last 10 years of my career at OGT in various functions of the business, I can say that the company really emphasizes the importance personal development, allowing staff to develop skills and expertise in areas of interest. In my commercial roles, I’ve had the opportunity to travel around the world and support the development of genomic testing services, which is incredibly rewarding!

Alex Hobbs
Account Manager UK, Ireland & Nordics
As an FAS at OGT, we are the front-line support who work with different departments in intimate collaborations. Our technical knowledge is constantly challenged and enriched, while our skillset on prioritization, reaching goals, and leading difficult conversations are always evolving. There are various positions an FAS can transition to as a next career step.

Huiyan Jin
Senior NGS FAS
First of all, we will ask you to provide us with a copy of your CV, along with a covering letter outlining your suitability for the role, salary expectations and confirming you have the right to work in the country.
Secondly, we will review each of the applications and the candidates who are most suited for the position, based on the skills, competencies and experience will be selected. If you are suitable, we will contact you and you will be invited to the first interview.
You may have a short telephone interview with general questions about the role for which you are applying. Be prepared to talk about why you are interested in the role and your motivations.
If your first interview has been successful, we will contact you and invite you to attend a face to face interview. This interview will be more in-depth and explore your skills and experiences in more detail. You will also have the opportunity to ask your own questions about the role.
If you are successful then we will contact you and offer you the position. We will confirm the terms and conditions of your employment in writing. We will also ask you for two references and we will look forward to you joining the team.
Regulation (EU) 2017/746 (IVDR) is a new regulatory framework for in vitro diagnostic (IVD) medical devices, which was introduced in 2017 to replace Directive 98/79/EC (IVDD). Following a 5-year transition period, IVDR became effective across the European Union (EU), European Economic Area (EEA) and Turkey on 26th May 2022. IVDR also applies to Northern Ireland because of the Northern Ireland Protocol to the Brexit withdrawal agreement.
The new Regulation brings revolutionary changes to the IVD industry. It sets higher standards for quality and safety for in vitro diagnostic medical devices (including in-house devices) to ensure the highest level of public health protection. By creating a more robust, transparent and sustainable regulatory framework, the IVDR will also ensure fairer market access for all diagnostic manufacturers.
Objectives of IVDR

The most significant change is the new risk-based classification system and the requirement that manufacturers of IVD devices must consult a Notified Body for approval (Class A non-sterile exempted). Under the new Regulation, self-declaration is no longer permissible for higher risk devices (Classes B-D, plus sterile Class A).
Successful transition to IVDR-certification requires manufacturers to prepare more stringent technical documentation related to scientific validity, analytical performance, clinical performance and risk management, in addition to conducting comprehensive post-market surveillance. To improve transparency and traceability, unique device identification (UDI) is required for every device, and these must be registered in EUDAMED, the new European database on medical devices.
The IVDR date of application was 26th May 2022.
From this date, any new (or significantly modified) IVD device placed on the market must comply with the IVDR.
For existing products already on the market, the EU recently granted an extension to the transition period, with the length of extension being dependent upon the risk class. CytoCell IVD FISH probes are categorised as Class C devices and as such need to be fully compliant by May 2026.
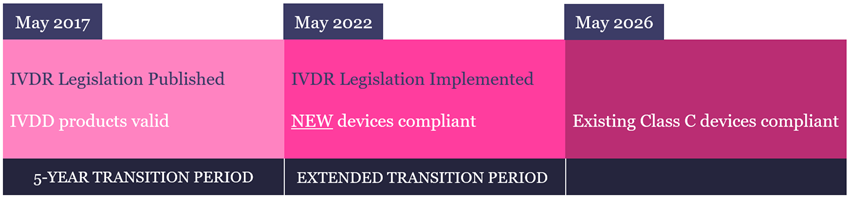
The IVDR will affect every FISH probe on the market as they must comply with the new Regulation by May 2026 or become RUO products.
Available now!
Sixteen (16) CytoCell FISH probes that play a critical role in the management of patients with haematological cancers and prenatal conditions have been IVDR-certified and are available to order:
Haematological malignancies
Prenatal testing
Evaluation kits
Uncertain whether your current FISH probe(s) will become IVDR-certified? Switch to a CytoCell IVDR FISH probe and be reassured that your chosen probe is already fully compliant. We can help you start the transition early by validating our IVDR-certified FISH probes - request your complimentary evaluation kit today!
Existing CytoCell customers
Current users of CytoCell IVDD FISH probes can be assured that our IVDR equivalent probe designs remain unchanged, so there is no requirement to re-validate your probes.
Further certifications
Additional IVDR-certified probes will follow over the coming months as we continue to pursue certifications for our CytoCell FISH probes. Be the first to know when new IVDR probes become available – sign-up today.
Revalidation
Hospital and commercial diagnostic testing labs that use CE-IVDR FISH probes according to the manufacturer’s published method, and for the Intended Purpose, are classified as “users”. There are no additional requirements for users provided that the laboratory does not change the Intended Purpose or method.
Many of OGT’s existing CE-IVDD FISH probes will transition to become IVDR-certified. The majority of the IVDR FISH probes will be identical to the IVDD version in terms of formulation, design, specification and Intended Purpose, so there is no requirement for additional validation work to be performed.
Health Institution Exemption for “In-House Devices”
IVDR also introduces a common set of rules for in-house devices that are manufactured and used within the same Health Institution. Although the use of in-house devices is largely prohibited under IVDR, there are exceptions provided that in-house devices meet certain safety and performance requirements.
Key requirements:
Find out more about these exemptions on the European Union website
EUDAMED
With the implementation of the IVDR, a new European Database on Medical Devices (EUDAMED) has been launched that will provide an overview of all commercially available devices CE marked under IVDR, including the specifications of the assay, limitations, performance and UDI information. This will enable laboratories to make a more informed decision on which device is best suited for their needs. Additionally, devices with Notified Body certification for IVDR will include the four-digit Notified Body identification number on the label for greater confidence and traceability.
customer partnerships
FISH, array & NGS products developed
years’ experience in molecular genetics
countries where our products are used
Watch our video for the probe application step of the haematology fluorescence in situ hybridisation (FISH) protocol.
Discover moreLorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Home






















