At OGT, we committed early to the In Vitro Diagnostic Regulation (IVDR) and have been working diligently over the last few years to meet the conformity requirements in a timely and compliant manner.
We’re delighted that this hard work has now led to the successful IVDR certification of sixteen (16) CytoCell® fluorescence in situ hybridisation (FISH) probes that play a critical role in the management of patients with haematological cancers and prenatal conditions.
This important milestone places OGT as the first manufacturer of FISH probes to obtain the new IVDR certification, demonstrating our commitment to provide innovative, class-leading products under this substantially more stringent regulation. Switching to IVDR-certified FISH probes means you can be confident that your laboratory is using safe, reliable and effective tools for diagnosing patients.
Further product launches will follow over the coming months as we pursue additional certifications for our CytoCell FISH probes.
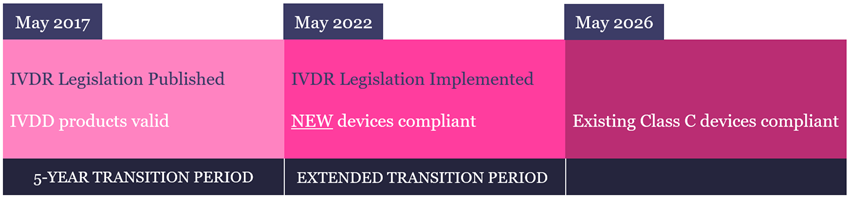
Regulation (EU) 2017/746 (IVDR) is a new regulatory framework for in vitro diagnostic (IVD) medical devices, which was introduced in 2017 to replace Directive 98/79/EC (IVDD). Following a 5-year transition period, IVDR became effective across the European Union (EU), European Economic Area (EEA) and Turkey on 26th May 2022. IVDR also applies to Northern Ireland because of the Northern Ireland Protocol to the Brexit withdrawal agreement.
The new Regulation brings revolutionary changes to the IVD industry. It sets higher standards for quality and safety for in vitro diagnostic medical devices (including in-house devices) to ensure the highest level of public health protection. By creating a more robust, transparent and sustainable regulatory framework, the IVDR will also ensure fairer market access for all diagnostic manufacturers.
Objectives of IVDR

The most significant change is the new risk-based classification system and the requirement that manufacturers of IVD devices must consult a Notified Body for approval (Class A non-sterile exempted). Under the new Regulation, self-declaration is no longer permissible for higher risk devices (Classes B-D, plus sterile Class A).
Successful transition to IVDR-certification requires manufacturers to prepare more stringent technical documentation related to scientific validity, analytical performance, clinical performance and risk management, in addition to conducting comprehensive post-market surveillance. To improve transparency and traceability, unique device identification (UDI) is required for every device, and these must be registered in EUDAMED, the new European database on medical devices.
The IVDR date of application was 26th May 2022.
From this date, any new (or significantly modified) IVD device placed on the market must comply with the IVDR.
For existing products already on the market, the EU recently granted an extension to the transition period, with the length of extension being dependent upon the risk class. CytoCell IVD FISH probes are categorised as Class C devices and as such need to be fully compliant by May 2026.
The IVDR will affect every FISH probe on the market as they must comply with the new Regulation by May 2026 or become RUO products.
Available now!
Sixteen (16) CytoCell FISH probes that play a critical role in the management of patients with haematological cancers and prenatal conditions have been IVDR-certified and are available to order:
Haematological malignancies
Prenatal testing
Evaluation kits
Uncertain whether your current FISH probe(s) will become IVDR-certified? Switch to a CytoCell IVDR FISH probe and be reassured that your chosen probe is already fully compliant. We can help you start the transition early by validating our IVDR-certified FISH probes - request your complimentary evaluation kit today!
Existing CytoCell customers
Current users of CytoCell IVDD FISH probes can be assured that our IVDR equivalent probe designs remain unchanged, so there is no requirement to re-validate your probes.
Further certifications
Additional IVDR-certified probes will follow over the coming months as we continue to pursue certifications for our CytoCell FISH probes. Be the first to know when new IVDR probes become available – sign-up today.
Revalidation
Hospital and commercial diagnostic testing labs that use CE-IVDR FISH probes according to the manufacturer’s published method, and for the Intended Purpose, are classified as “users”. There are no additional requirements for users provided that the laboratory does not change the Intended Purpose or method.
Many of OGT’s existing CE-IVDD FISH probes will transition to become IVDR-certified. The majority of the IVDR FISH probes will be identical to the IVDD version in terms of formulation, design, specification and Intended Purpose, so there is no requirement for additional validation work to be performed.
Health Institution Exemption for “In-House Devices”
IVDR also introduces a common set of rules for in-house devices that are manufactured and used within the same Health Institution. Although the use of in-house devices is largely prohibited under IVDR, there are exceptions provided that in-house devices meet certain safety and performance requirements.
Key requirements:
Find out more about these exemptions on the European Union website
EUDAMED
With the implementation of the IVDR, a new European Database on Medical Devices (EUDAMED) has been launched that will provide an overview of all commercially available devices CE marked under IVDR, including the specifications of the assay, limitations, performance and UDI information. This will enable laboratories to make a more informed decision on which device is best suited for their needs. Additionally, devices with Notified Body certification for IVDR will include the four-digit Notified Body identification number on the label for greater confidence and traceability.
IVDR is all about patient safety and effectiveness, and at OGT, we’re really committed to compliance with changing worldwide regulations and providing products that meet these needs, for clinicians and patients alike. We are 100% ready, having gained certification for sixteen (16) CytoCell FISH probes. Our IVDR FISH probes are still the same trusted products that we’ve always had—the certification has further validated our quality, safety and effectiveness. These are products and a company you can depend on.

Steve Chatters
Executive Vice President of Regulatory, Medical and Quality Affairs at OGT
