The results of therapeutic clinical trials reported at the American Society of Hematology (ASH) 2023 Annual Meeting reveal opportunities to re-imagine the role of well-established cytogenetic and molecular biomarkers in guiding the treatment of haematological malignancies.
An undoubted highlight among the thousands of presentations at this year’s conference in San Diego, California has been the emergence of menin inhibitors as a promising new class of therapeutics for the treatment of acute leukaemia defined by KMT2A rearrangements (KMT2Ar) or mutations in NPM1. Both mutations are recognised as defining genetic abnormalities in AML by the World Health Organization.1
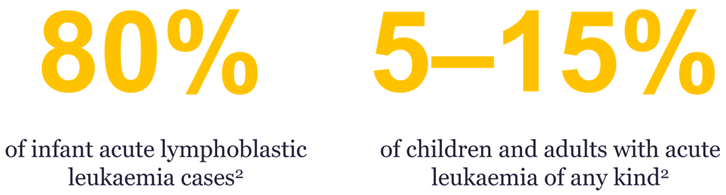
In the case of patients with KMT2Ar, these new investigational therapeutics bring the promise of precision medicine to patients with subtypes of leukaemia that have hitherto had a dismal prognosis and limited chemotherapeutic treatment options, including chemotherapy resistance and high rates of relapse.3
Menin inhibitors block the interaction between menin and KMT2A fusion proteins that are critical to driving leukemogenesis in KMT2Ar patients. Several presentations across the ASH ’23 programme showed data from Phase I and II clinical studies suggesting these investigational therapeutics are highly effective at inducing remission in relapsed or refractory disease, and in some cases even allow patients to recover enough to proceed to stem cell transplantation.
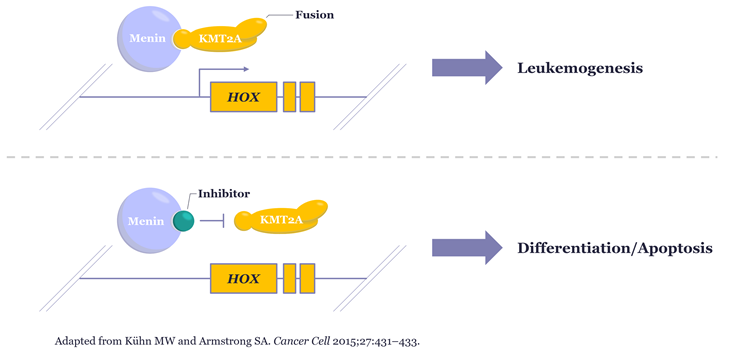
Given these exciting results, there is renewed focus on how best to identify patients who could be eligible for enrolment in future clinical trials of menin inhibitors, including those testing their effectiveness as part of first line combination therapy, however, as KMT2A has a bewildering array of over 80 known fusion partners, resulting from many potential breakpoints across this large gene, this identification can be challenging. Some traditional approaches can struggle to deliver sufficiently timely, accurate and cost-effective detection of the full range of clinically relevant rearrangements.
Fortunately, cytogeneticists can use breakapart FISH probes that are agnostic to the fusion partner to overcome these limitations. Even better news is that breakapart FISH testing of KMT2A is already a mainstay of AML cytogenetic detection, and companies, including ourselves at OGT, already manufacture IVDR approved assays for this purpose.
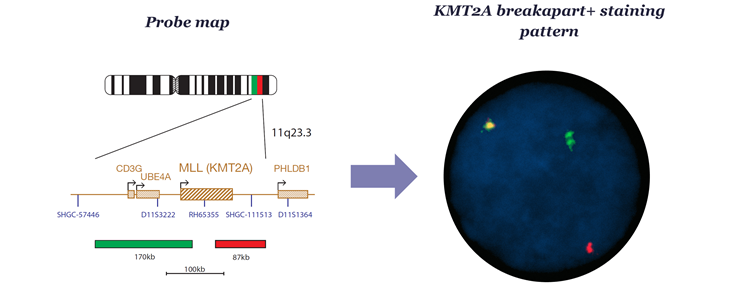
Any future use of these assays as investigational tools to determine eligible patients for clinical trials, or even ultimately as companion diagnostics, would require further validation and regulatory review. However, we are nevertheless optimistic that the field of cytogenetics, where we at OGT are proud to play a key role, would be well placed to support a change in how we use KMT2Ar tests should this be required in the coming years. In the meantime, we watch with excitement trials of these therapeutics as part of combination regimes for first line treatment and hope for a future where a finding of KMT2Ar on a cytogenetic report is no longer just a marker of ‘adverse risk’ but is also a marker of hope for patients.
See how you can leverage OGT’s clinical expertise to ensure you maximise your IVDD and IVDR cytogenetics workflow efficiency here: www.ogt.com/ivdr
Note: IVDR-approved probes are only available in Europe and selected other countries.

In the second part of our latest blog series - “Old biomarkers, new tricks" - we highlight the ongoing efforts to bring BCL-2 inhibitors to the forefront in t(11:14) translocation cases of multiple myeloma, and how FISH probes can support clinical investigations.
Read
In March 2023, we became the very first manufacturer of FISH probes to be granted IVDR certification. In our latest blog we speak with Steve Chatters, Executive Vice President of Regulatory, Medical and Quality Affairs, about OGT's long-standing expertise in healthcare regulation, and the journey towards IVDR certification.
Read