Want to download this as a PDF? Download now
Graham Speight1, Ephrem Chin1, Jacqueline Chan1, Robert Zeillinger2, Nicole Concin3, David Cook1
1Oxford Gene Technology, Oxford, UK; 2Medical University of Vienna, Dept. of Obstetrics and Gynaecology, Vienna, Austria and 3Medical University, Dept. of Gynaecology and Obstetrics, Innsbruck, Austria
One of the challenges in the treatment of cancer is the high level of genetic complexity and tumour heterogeneity. Detailed information about the genetic profile of each individual tumour can help guide treatment strategies1. Epithelial ovarian cancer (EOC) is the most lethal gynaecological malignancy2 , and the type II tumours accounting for approximately 75% of all EOCs, are nearly always detected in advanced stages. These highly aggressive tumours are characterised by their morphological and molecular homogeneity and often (>80% of cases) contain TP53 mutations. The GANNET53 (Ganetespib in metastatic, p53 mutant, platinum-resistant ovarian cancer) trial started in October 2015 and aims to improve the prognosis and quality of life in platinum-resistant EOC patients (www.gannet53.eu). This European multi-centre clinical trial is currently in stage II during which biomaterials have been collected and analysed using a SureSeq™ hybridisation-based enrichment panel for targeted next-generation sequencing (NGS), to determine the TP53 mutation status of the samples.
Multiplex PCR-based approaches are often used to analyse low input DNA from FFPE samples. However, they are not able to (without molecular barcodes) fully elucidate the true allele frequency and complexity, which is essential to fully evaluate highly heterogenic tumour samples. Hybridisation-based enrichment approaches typically demonstrate better uniformity, are more likely to preserve the complexity of the original sample, are more tolerant of both DNA quality and unknown variants in the capture region3.
![]() Table 1: Performance comparison of amplicon- and hybridisation-based capture methods.
Table 1: Performance comparison of amplicon- and hybridisation-based capture methods.
Genetic profiling of solid tumours is often problematic as tissue biopsies are typically archived as formalin-fixed, paraffin-embedded (FFPE) blocks, which preserve tissue morphology and permits long-term storage at room temperature. However, the methods used for fixation significantly damage and compromise the quality of nucleic acids in these samples. Formalin damage can fragment the DNA which can impair PCR (a required process in NGS library preparation). Consequently, library yields and the ability to obtain high-quality, meaningful sequence data are compromised, affecting the confident identification of variants.
We tested a range of FFPE derived DNA and found treatment with the SureSeq FFPE DNA Repair Mix significantly improved the mean target coverage, thereby increasing the confidence of the variant calls (Figure 1A). Use of the Repair Mix also enabled a reduction in the amount of DNA input down to 50 ng whilst maintaining good depth of coverage (Figure 1B).
 Figure 1: Example data obtained using FFPE DNA extracted from Ovarian tumour samples. Panel A shows that the SureSeq FFPE DNA Repair Mix improves on-target reads (OTR); Panel B demonstrates the use of lower DNA inputs whilst maintaining depth of coverage.
Figure 1: Example data obtained using FFPE DNA extracted from Ovarian tumour samples. Panel A shows that the SureSeq FFPE DNA Repair Mix improves on-target reads (OTR); Panel B demonstrates the use of lower DNA inputs whilst maintaining depth of coverage.
All 32 samples presented here were provided by the GANNET53 trial and contained 40% tumour content. DNA was extracted from tissue curls using standard methods. The resultant DNA was analysed using the hybridisation-based SureSeq Ovarian Cancer Panel and subsequently sequenced on an Illumina MiSeq®. Each sample received between 1/10th to 1/16th of a lane. The average depth of coverage (after removal of PCR duplicates) over the seven target genes (ATM, ATR, BRCA1, BRCA2, NF1, PTEN and TP53) was 666. We confidently detected one or more deleterious TP53 variants in all 32 samples with the minor allele frequencies (MAF) ranging from 1.1 – 79.6%.
In addition to the mutations in TP53, 22 of the samples were found to have additional variants in BRCA1 and BRCA2, (Integrated Genome Viewer [IGV4] images of examples are shown in Figures 2 & 3), of which 8 were likely germline (defined as having a MAF between 45-55% or >95%). The remaining putative somatic variants had MAFs ranging from 2.3 to 71.3%. Variants were also found in ATM in 5 of the mutant TP53 tumour samples (example shown in Figure 4).
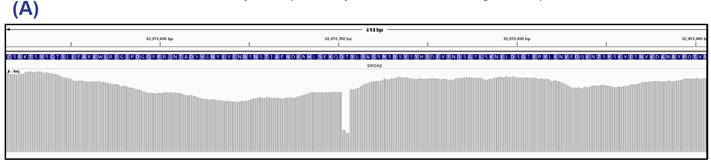
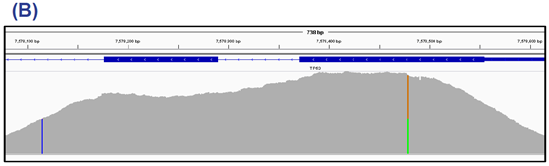 Figure 2: BRCA2 exon 11 (panel A) and TP53 exon 5 (panel B). This sample contains a 46% Pro151Ser SNV in TP53 as well as a four nucleotide deletion with a 70% allele frequency in BRCA2.
Figure 2: BRCA2 exon 11 (panel A) and TP53 exon 5 (panel B). This sample contains a 46% Pro151Ser SNV in TP53 as well as a four nucleotide deletion with a 70% allele frequency in BRCA2.
 Figure 3: Example coverage of BRCA1 exon 10. The whole exon (3425 bp) is covered uniformly allowing confident detection of nine variants, each of 60% allele frequency. This sample also had a 34% Arf273His mutation in TP53 (data not shown).
Figure 3: Example coverage of BRCA1 exon 10. The whole exon (3425 bp) is covered uniformly allowing confident detection of nine variants, each of 60% allele frequency. This sample also had a 34% Arf273His mutation in TP53 (data not shown).
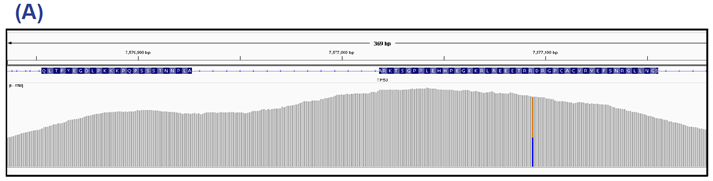
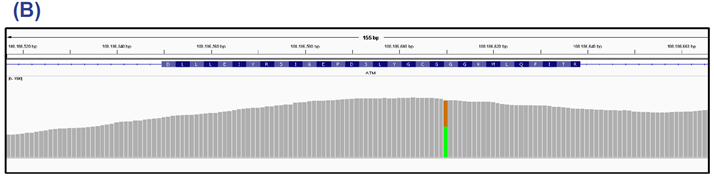 Figure 4: TP53 exons 9 and 10 (panel A) and ATM exon 41 (panel B). This sample contains a 68% nonsense mutation in TP53 and 53% Gly2023Arg SNV in ATM.
Figure 4: TP53 exons 9 and 10 (panel A) and ATM exon 41 (panel B). This sample contains a 68% nonsense mutation in TP53 and 53% Gly2023Arg SNV in ATM.
The SureSeq hybridisation-based workflow was used to determine the genetic profile of 32 ovarian tumours. The workflow of this approach is outlined in Figure 5.
 Figure 5: OGT SureSeq workflow. The SureSeq Ovarian Cancer panel targets seven key genes – ATM, ATR, BRCA1, BRCA2, NF1, PTEN and TP53.
Figure 5: OGT SureSeq workflow. The SureSeq Ovarian Cancer panel targets seven key genes – ATM, ATR, BRCA1, BRCA2, NF1, PTEN and TP53.
SureSeq: For Research Use Only; Not for Diagnostic Procedures.
