In the 23 years since the first approval of a targeted therapy (Gleevec)1 in chronic myeloid leukemia, the implementation of precision medicine across hematological malignancies has been dramatic but fragmented.
Patients with acute or chronic leukemia can now benefit from several therapies targeted to the specific genomic aberration that defines their subtype of disease, with a number more in advanced stages of development (see the Part 1 blog on AML here). In contrast, there is not a single approved precisely targeted therapy for patients with multiple myeloma (MM), an incurable condition which can cause abnormal plasma cells to form tumors within the body and produce unneeded plasma proteins.2

News of potential new treatment modalities for MM, including CAR-T therapies and bi-specific antibodies, was abound at this year’s ASH meeting. However, as yet these prospective therapeutics are not being accompanied by biomarker testing which would aid in identifying which MM patients would most likely benefit from their use, or indeed be most susceptible to their possible toxicities.
Through the routine use of comprehensive FISH panel testing at diagnosis it is possible to distinguish between subgroups of MM patients which may have widely varying prognoses, and in some cases be associated with differences in underlying disease biology. The relevance of these diagnostic cytogenetic biomarkers has not yet been shown for targeting newer classes of biological therapy in MM.
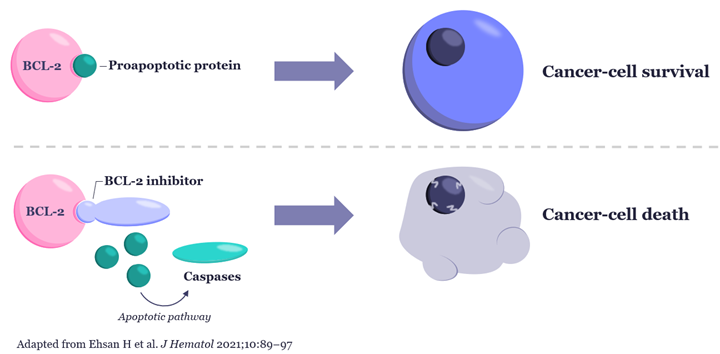
However, there is promising news of their potential utility in the identification of patients who may benefit from the next generation of a well-established drug class used in treating a number of hematological malignancies, BCL-2 inhibitors.
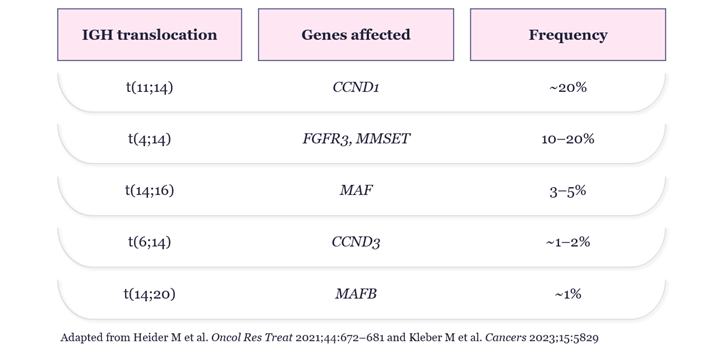
For many years it has been recognized that the 20% of patients whose MM is defined by t(11;14) translocations are more likely to over express the anti-apoptotic protein BCL-2, a key driver of malignant cell proliferation.5 Thus, t(11;14) was identified as a biomarker for patients who may benefit from BCL-2 inhibition but was followed by the recent disappointment of venetoclax, a first generation BCL-2 inhibitor failing in a Phase 3 trial of t(11;14) positive MM patients.6
The MM research and clinical community remains convinced that the BCL-2 pathway could and should be targeted in these patients. This confidence was reinforced by early Phase 1b/2 results presented at the ASH 2023 meeting of second generation BCL-2 inhibitors, which may have higher potency and/or better safety profiles. In particular, results presented from the Phase 1b/2 study of sonrotoclax in t(11;14) patients showed promising rates of response, albeit in a small dose escalation cohort.7
For patients with t(11;14) positive malignancies there is certainly hope that better, more targeted therapies may be on the way in the coming years. Of course, there is further work to be done in randomized, controlled late stage trials to determine the efficacy of second generational BCL-2 inhibitors. Nevertheless, patients and clinicians can be reassured that diagnostic companies, including OGT and our partners in cytogenetics laboratories around the world, are well placed to support future development and implementation requirements.
OGT is committed to continuous improvement of our CytoCell® IVD FISH probe panels, including multiple myeloma panels that detect t(11;14) translocations, to ensure they remain compliant with the most stringent regulatory expectations, including IVDR, and we are ready to support their use in both clinical trials and, in the future, as companion diagnostics.
View our complete portfolio of CytoCell FISH probes for hematological, solid tumor and constitutional cytogenetics here: www.ogt.com/cytocell
Note: IVDR-approved probes are only available in Europe and selected other countries.

Didn’t get a chance to talk to us at ASH 2023? Read the first part of our latest blog series, which describes the potential of menin inhibitors for acute leukemia, including the use of IVDR approved FISH probes in clinical trials.
Read
In March 2023, we became the very first manufacturer of FISH probes to be granted IVDR certification. In our latest blog we speak with Steve Chatters, Executive Vice President of Regulatory, Medical and Quality Affairs, about OGT's long-standing expertise in healthcare regulation, and the journey towards IVDR certification.
Read