Oxford, UK – 25 April 2017. OGT, The Molecular Genetics Company, has expanded its SureSeq myPanel™ NGS Custom Cancer Panel content. The expansion comes in response to NGS becoming ever more important for research into an increasing number of cancer types, and a need to obtain reliable data for difficult-to-sequence genes and mutations. The expanded content now covers over 70 genes, with many more available in the near future, optimized for hematology and solid tumors including breast, colorectal, lung, ovarian and prostate cancer, glioma, melanoma, sarcoma, leukemias, myeloproliferative neoplasms and myelodysplastic syndrome.
The strength of OGT’s hybridization-based bait design coupled with its unique expertise delivers excellent whole gene coverage, unparalleled uniformity and the ability to sequence even the most difficult of regions. This yields reproducible and meaningful data, enabling researchers to be confident in sequencing results and analyses.
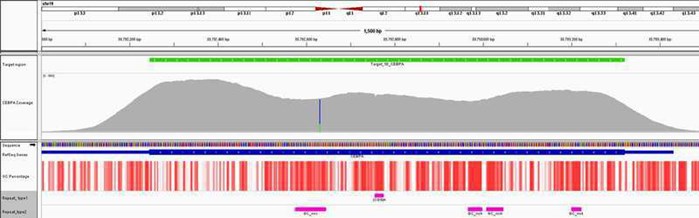
Fig 1. Excellent uniformity of coverage of the CEBPA gene averaging ~3000x coverage. Depth of coverage per base (grey). Targeted region (green). Gene coding region (blue). GC percentage (red). Repeat regions and GC-rich regions (pink).
Some of the most difficult cancer-associated genes to sequence are those that have high levels of GC-rich content, such as the tumor suppressor gene CEBPA in acute myeloid leukemia, and TP53, which is frequently mutated in many cancer types including breast cancer. In addition, genes that contain internal tandem duplications are challenging to target due to their repetitive nature and length. Panels available from other suppliers can experience problems with data variability, coverage drop-out or masked mutations in these regions. In contrast, OGT’s innovative bait design uniquely overcomes these issues, delivering the high levels of uniform coverage required, and reducing the need for supplementary fill-in by Sanger sequencing.
This unique design allows researchers to detect low frequency variants consistently down to 1% VAF (variant allele frequency) at a read depth of >1000x. The panels are fully customizable—researchers can select the gene, exonic or intronic content needed to create an NGS cancer panel that meets their exact requirements. All the content is fully pre-optimized, removing the need for lengthy in-house optimization, reducing assay development time.
Dr Anna Skowronska, R&D Scientist at West Midlands Regional Genetics Laboratory, has been using the SureSeq Myeloid Panel content. She commented: “We’ve found that the SureSeq Panel performs very well and has detected all known positives with an excellent variant detection of around 1%... we were positively surprised right from the very first analysis by the level of mutation detection achievable.”
OGT closely collaborates with leading experts and examines the current literature to provide the most up-to-date targeting of all relevant regions. This includes exonic, intronic and splice sites, and delivers comprehensive insight into disease-driving mutations. Dave Cook, Senior Product Manager at OGT elaborated: “We’re continually working to expand our custom NGS panel content to ensure we have the most recent and relevant content. Our coverage and reproducibility is unrivaled in the market and we wanted to expand the accessibility of quality NGS data to a wider range of cancer types—including genes and regions that are known to be difficult to sequence. We’re committed to helping customers reach their goals and we’re pleased to report that our expertise has enabled us to overcome these sequencing difficulties, giving them more confidence in the data they are generating”.
About OGT
OGT, a Sysmex Group company, is a leading global provider of clinical and diagnostic genomic solutions that are created for scientists by scientists - including CytoCell®, CytoSure® and SureSeq™ ranges of FISH, microarray and NGS products. The company is dedicated to creating products that enable researchers and clinical decision makers to reach the right care decisions for each patient, every time. OGT strives to unlock the future of genetic clinical care with a commitment to working in partnership with its customers - not only by sharing its expertise of 25 years at the forefront of genetic endeavour, but also by working closely with scientists to understand their unique challenges, and to customise its approach to meet their exact needs. Dedicated to improving clinical care, OGT believes that through partnership—together—we’ll achieve more.
For more information on the Company, please visit our website at ogt.com
CytoSure®, SureSeq™ and myProbes®: For Research Use Only, not for use in diagnostic procedures. CytoCell: Some products may not be available in your region.
About Sysmex Corporation
Sysmex Corporation is a world leader in clinical laboratory systemization and solutions, including laboratory diagnostics, laboratory automation and clinical information systems. Serving customers for more than 50 years, Sysmex focuses on technological leadership in diagnostic science and information tools that make a difference in the health of people worldwide. The company is also exploring emerging opportunities in the life science field. Its R&D efforts focus on the development of high-value-added testing and diagnostic technologies that are innovative, original and optimize individual health. Sysmex also seeks to leverage its state-of-the-art technologies for cell, gene and protein analysis. The company, headquartered in Kobe, Japan, has subsidiaries in North America, Latin America, Europe, the Middle East, Africa, China and Asia Pacific and employs more than 9,000 employees worldwide. Sysmex Corporation is listed in the top tier of the Tokyo Stock Exchange.
For more information about Sysmex Corporation and its affiliate companies, please visit www.sysmex.co.jp/en/.

In the workshop users will explain how OGT’s SureSeq™ NGS panels can increase throughput and save time and cost in the detection of a wide variety of aberrations.
Read
Launch of two NGS panels enables comprehensive detection of genetic abnormalities involved in breast and ovarian cancer, and myeloid disorders.
Read
New content and Interpret NGS analysis software detection capabilities include BCR-ABL and KMT2A-PTD detection.
Read