Welcome to the latest edition of DNA Dispatch, the quarterly newsletter from OGT, your hybridization experts.
The collaboration expands OGT’s bioinformatics capabilities by integrating QIAGEN’s advanced tertiary analysis solution, QCI Interpret, with its SureSeq™ NGS panels.
As a result, SureSeq NGS panel users will gain access to streamlined, high-confidence genomic insights and efficient, scalable reporting from sample to result.
We’re proud to announce that U.S. FDA has granted marketing authorization for our first Companion Diagnostic (CDx) — the CytoCell® KMT2A Breakapart FISH Probe Kit PDx* — which supports Syndax Pharmaceuticals, Inc.'s REVUFORJ®.
To address current limitations in MRD testing methods, clinicians and diagnosticians are adopting more expansive genomic technologies—such as NGS—to capture the broader range of genomic biomarkers associated with blood cancer MRD.
Hear the experts at OGT discuss why today NGS is being used more widely to detect MRD in AML.
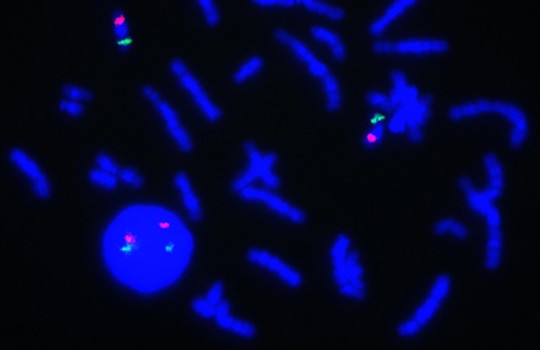
Fluorescence in situ hybridization (FISH) is a cytogenetic technique that can detect specific sites of DNA sequences in metaphase or interphase cells.
Watch OGT’s most recent video to learn more about the different chromosomal aberrations detected by FISH.
The aim of this study was to explore whether a NGS assay could deliver a comprehensive approach for the identification of multiple types of genomic drivers.
To do this the laboratory utilised a SureSeq myPanel™ Custom NGS Panel.
September was Blood Cancer Awareness Month, and throughout the month OGT colleagues took part in various events to help raise money and awareness.
To end the month we created a blood cancer quiz with the aim of increasing people's knowledge of this complicated group of cancers.
Don’t forget…Our FAS team can be contacted online with free support advice
* For In Vitro Diagnostic Use. Rx Only. For indications, limitations and other information, see the device package insert or go to www.ogt.com/kmt2a-pdx
† Source: https://www.bcam.org.nz/