A liquid biopsy is a broad term used to describe the collection and analysis of non-solid biological tissues derived primarily from peripheral blood. A liquid biopsy assay detects and analyses relevant tumor biomarkers utilizing mainly circulating tumor DNA (ctDNA) or circulating tumor cells (CTC)1 as the sample material. Analysis of these tumor-driven biomarkers may assist in cancer detection, treatment selection, therapy response monitoring, and studying resistance mechanisms.
In recent years, liquid biopsy has gained traction because it provides easy access to tumor-derived biomarkers with fewer potential complications for the patient compared to a surgical biopsy.
In 2016, the US-FDA approved the first liquid biopsy to aid in the detection of specific mutations in the epidermal growth factor receptor (EGFR) gene in non-small cell lung cancer (NSCLC) patients. These mutations may predict response to EGFR tyrosine kinase inhibitors1. Today, multiple commercial liquid biopsy assays have been approved in various oncological applications.
While liquid biopsy may present an attractive alternative to a solid biopsy, it also has limitations. Here, we shed light on some advantages, limitations, and future outlook for liquid biopsy in oncology clinical practice.
Solid biopsies remain the gold standard and a routine test in today's clinical practice. They enable the histological evaluation of a tumor, provide tumor staging and identify actionable genetic alterations to guide patient care. However, solid biopsies also have their limitations. First, a solid biopsy is invasive and is, in certain tumors, hard to obtain. Furthermore, a single solid biopsy might not provide a comprehensive picture of the tumor's genetic landscape, as many tumors are morphologically and genetically heterogeneous with numerous subclones.
In these cases, a liquid biopsy assay may provide an alternative and assist in identifying key genetic alterations that aid in diagnosing, treating, and monitoring periods of relapse and remission in cancer patients. Additionally, a liquid biopsy assay is easily repeated and may provide indications of ongoing metastasis and insight into the aggressive disease state.
Liquid biopsy is still a nascent technology with numerous challenges that hinder its incorporation into standard clinical practice. One disadvantage of a liquid biopsy assay is that it may miss critical genetic alterations in early disease states simply because ctDNA from a particular tumor may exist in extremely low concentrations. This may result in a delay in the diagnosis and administration of crucial life-saving therapeutics.
Furthermore, in some cases, age-related clonal hematopoiesis of indeterminate potential (CHIP) can interfere with ctDNA testing and cause incorrect interpretation of results, leading to inappropriate therapeutic decisions for patient management.
Precision medicine has revolutionized patient care and allowed a personalized approach to targeted therapeutics such as tyrosine kinase inhibitors (TKI) and immunotherapies2. These therapeutics often rely on detecting genetic alternation in tumor cells, and liquid biopsies offer real-time and easy access to the evolving tumor landscape. However, Liquid biopsies may still lack widespread clinical and commercial acceptance.
In addition, a solid biopsy will be needed for the initial histological diagnosis of a tumor. Therefore, it is unlikely that liquid biopsy will replace solid biopsy for the foreseeable future but rather be used in conjunction with a solid biopsy to refine diagnosis and monitor response to treatment.
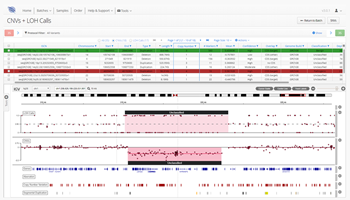
As one of the first steps in many NGS data analysis pipelines, accurate variant calling is often critical to downstream analysis and interpretation. Here, we take a look at variant calling best practice through a modern lens.
Read
A high-quality sequencing library is the linchpin to generating good sequencing data. We discuss our six top tips to help you improve your sequencing library.
Read
We discuss the development and current state of sequencing technologies, and where the future of NGS may take us...
Read
FISH is a cytogenetic technique utilized in labs to detect chromosomal abnormalities in both cancer and constitutional specimens. In this blog learn about the advantages of FISH...
Read
This blog will discuss FLT3’s normal function, its implications in myeloid malignancies, and the role of NGS in genetic identification and disease management of patients with FLT3 genetic alterations.
Read
Find out about the benefits and differentiating factors of the three most commonly used NGS technologies; targeted gene panels, whole-exome sequencing (WES) and whole-genome sequencing (WGS).
Read
Next generation sequencing (NGS) is now in routine use for a broad range of research and clinical applications. Facilitating the detection of a wide variety of mutations, focus has never been higher on the value of making the correct choice for the initial sequence enrichment step, which, if poorly designed, can be a source of bias and error in the downstream sequencing assay.
Read